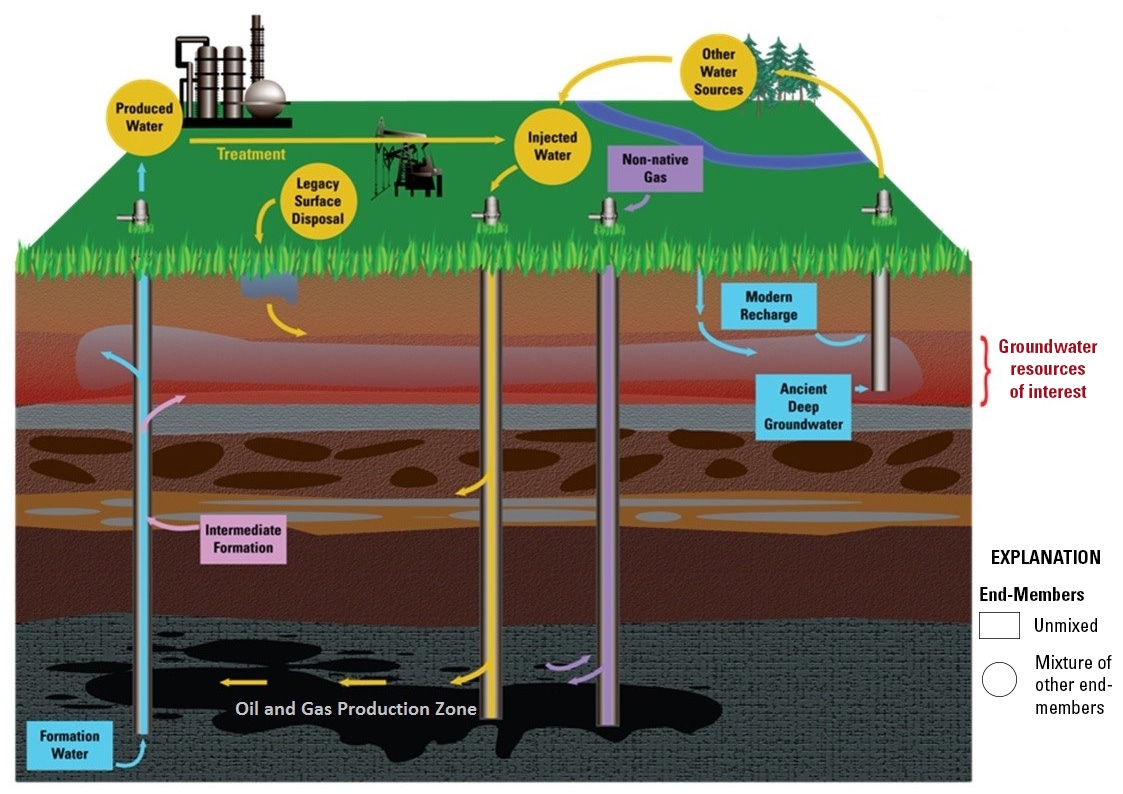
California Oil, Gas, and Groundwater Program
Geochemistry Library and End-Member Mixing Analyses
The COGG program uses geochemical principles to determine whether constituents found in groundwater samples come from oil and gas fluid sources and which pathways are likely responsible for fluid migration. This section describes how those principles are being applied in more detail.
End-Member Mixing Models
Chemical analyses of “end-member” samples will enable scientists to determine how much of each end-member source is present in groundwater samples. This end member mixing analysis is a common geochemical approach that uses the difference in chemical properties of environmental samples so man-made tracers do not have to be introduced (Christophersen and Hooper, 1992).
The chemical compositions of end-members can vary in time and space. The regional groundwater monitoring is conducting a review of well records to identify historical water chemistry data that have not yet been compiled in summary reports. Such data could allow one means of testing what changes may have occurred in oil field fluids over time. Variations in space, such as salinity concentrations within formations, are being examined using multiple tools and data sets.
Analytical Constituents
There is a large body of literature that provides insight regarding constituents that are useful tracers of oil and gas activities, including Jackson and others (2013), Orem and others (2014), Vengosh and others (2014); and Izbicki and others (2005). More recent studies focus on potential mobilization of fluids from geologic formations bearing shale gas in the eastern and central United States through unconventional oil and gas development techniques (Warner and others, 2014; Vengosh and others, 2013).
Based on that literature, the following constituent groups are being used in the analysis of groundwater and oil-field fluids for the regional groundwater monitoring:
A suite of dissolved hydrocarbons and other VOCS determining concentrations using the low-level detection methods.
- The VOCS include constituents that could be associated with well-stimulation, waste-injection activities, or naturally occurring hydrocarbon deposits.
Dissolved short-chain hydrocarbon gases (one to six carbons in a molecule), determining their concentrations, proportions, and isotopic ratios.
- These properties of short-chain hydrocarbons are diagnostic for determining the source of the gases, whether thermogenic (oil and gas sources) or biogenic (from microbial processes in aquifers), and in combination with other data can provide a means of identifying if hydrocarbon gases in groundwater are a result of leaking well casings.
Dissolved inorganic constituents, including major and minor ions and trace elements.
- Concentrations of these salts and metals, and the ratios of selected constituents can be used for determining sources of salinity in groundwater, which is necessary to distinguish the effects of oil and gas activities from other anthropogenic and natural sources of salinity in groundwater.
Dissolved noble gases are used to determine if isotopic values and ratios of these gases in a water sample reflect atmospheric or deep subsurface sources, and can, for instance, be used to identify water samples affected by fluid injection.
- In addition, a combination of dissolved noble and atmospheric gases can be used to calculate recharge location, and subsurface flow regimes. Concentrations and isotopic values of these tracers are essential ancillary data used to understand the relation of water quality to groundwater flow within an aquifer system.
Isotopic tracers, such as stable isotopes of water, dissolved carbon, strontium, boron, and lithium,
- Used for determining sources of water and salts and to calculate groundwater ages.
Naturally occurring radioactive materials.
- Can be effective tracers of fluids from zones of oil and gas deposits.
Semi-volatile organic compounds, including PAHS.
- Potential indicators of dissolved petroleum products in groundwater.
Organic carbon characteristics, including optical characteristics and separation of dissolved organic carbon fractions on the basis of polarity and molecular weight.
- Yield different geochemical signatures when the organic compounds come from petroleum sources compared with other sources of organic carbon.
Field parameters including pH, water temperature, specific conductance, turbidity, alkalinity, dissolved oxygen, and dissolved sulfide.
- Provide foundational information on geochemical conditions that affect the mobility of many constituents.
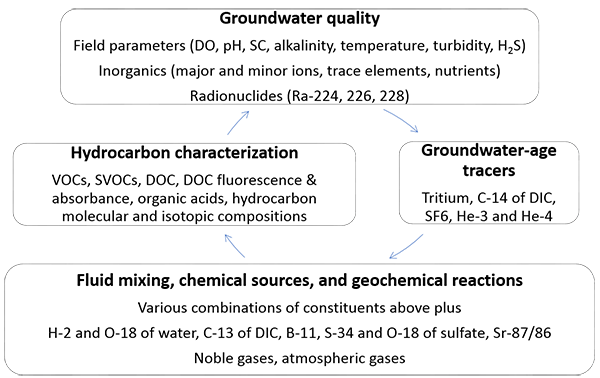
Constituents for regional groundwater monitoring, grouped by function.
Abbreviations:
DO - dissolved oxygen
pH - potential of hydrogen
SC - specific conductance
H2S - hydrogen sulfide
Ra - radium
VOCs - volatile organic carbons
SVOCs - semivolatile organic carbons
DOC - dissolved organic carbons
DIC - dissolved inorganic carbon
SF6 - sulfur hexafluoride
He - helium
H - hydrogen
O - oxygen
Sr - strontium
Exploratory Sampling
Exploratory sampling was conducted to determine whether the analytical suites of constituent groups reported in the literature could be used successfully in California oil fields to determine sources and pathways (McMahon and others, 2016). This section summarizes information for selected constituents from that report.
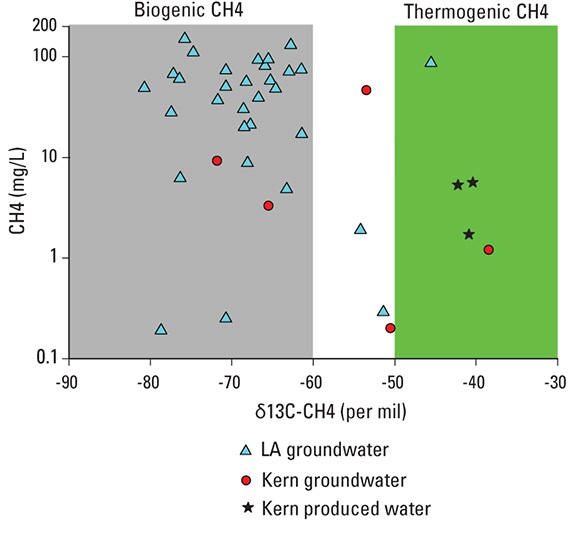
In the first graphic, methane concentrations are plotted against carbon isotopic compositions of methane. Past research has determined that points falling in the gray box represent methane that is derived from biological sources, and points falling in the green box represent methane derived from heated sources and thus oil and gas deposits.
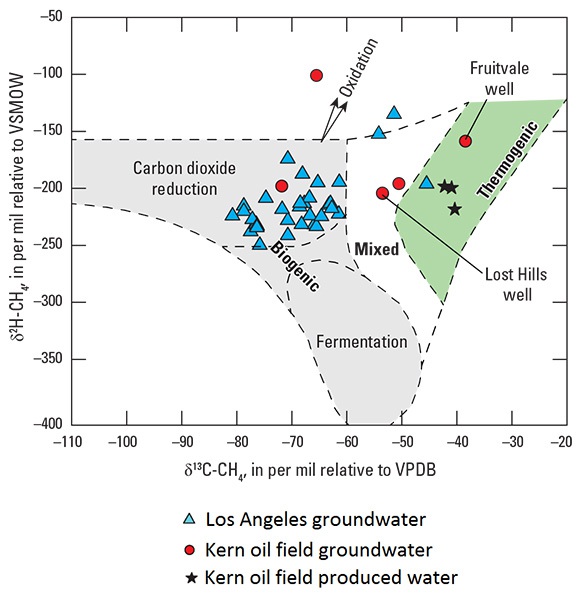
A second analysis is needed to look more carefully at the three triangles plotting outside the gray box. That analysis resulted in the second figure, showing carbon isotopic composition of methane plotted against hydrogen isotopic composition of methane. Using this information, the three samples were determined to plot differently than the rest because oxidation had taken place, not because they were mixed with thermogenically derived methane (McMahon and others, 2016).
The results given above are examples of the kinds of approaches that can be used to interpret geochemical data. Multiple tracer approaches were needed to recognize the presence of different chemicals from oil-field formations in groundwater. Formation waters were found to have complex chemical patterns that could not be generalized across different fields, meaning that monitoring for these fluids requires a suite of analytes that is robust enough to detect all of these complex patterns. A more limited analytical suite would fail to document connections between produced water and groundwater quality (McMahon and others, 2016).